Data Integrity Sop in Pharmaceutical Industry
The US-FDA uses the term ALCOA which refers to complete consistent and accurate data that should be Attributable. Good data management practices influence the integrity of all data generated and recorded by a manufacturer and these practices should ensure that data is accurate complete and reliable.
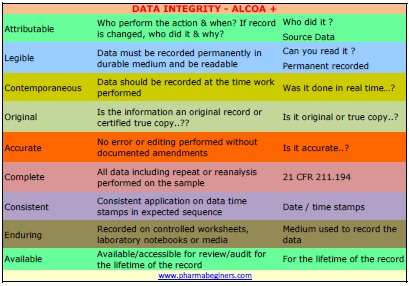
Data Integrity Handling Of Di Observations Dio Pharma Beginners
Data governance policy should be effectively endorsed at the.

. Data Integrity is the extent to which all data are complete consistent. Pharmaceutical Guidanace November 3 2018 Audit and Guideline Comments Off 2322 Views. Any issue in data integrity must be handled as per the quality management system QMS and proper corrective and preventive action CAPA must be taken according to risk assessment.
And the increased complexity of supply chains and. SOP on Data Integrity in Pharmaceutical Industry Part 1 0442 Part 2 0450 Student Ratings Reviews 45 Total 4 Ratings 5. Data Integrity shall be maintained in all manual or system generated electronic data.
Data integrity is important for ensuring the quality of drug products demonstrating compliance with regulatory. Principles of Data Integrity DI 1. Any identified data integrity issue shall be handled as per the quality management system and proper corrective and preventive action shall be taken according to risk assessment.
Ad Enables the QC data management process to go uninterrupted maintaining QC data integrity. What are the goals of data integrity. In the pharmaceutical industry ensuring data integrity involves generating and documenting data accurately protecting data from accidental or intentional modifications falsification deletion or destroying data.
12 months ago. The ALCOA principles were created by the FDA and are. The primary aim of data integrity is to protect patients.
Very good elaborated training. MHRA saysThe way regulatory data is generated has continued to evolve in line with the ongoing development of supporting technologies such as the increasing use of electronic data capture automation of systems and use of remote technologies. Key Takeaways Data integrity means that data are accurate complete consistent and validboth in the physical and logical sense.
Data Integrity in the Pharmaceutical Industry Meet GOFIVE and Paperless Validation M otivated by an increase in the number of nonconformities in the Pharmaceutical Industry regulatory agencies FDA MHRA OMS and PICS have issued guidance in recent years on how to keep intact critical data related to the production of their products. By Esco Healthcare 07 January 2019. Records and Data Integrity 2017 MHRA Guidance on GxP Data Integrity 2018 Only when data is reliable can business owners make the best suitable choices for their organizations improve the quality of.
What is Data Integrity In Pharmaceutical Industry. Standard Operating Procedure SOP for Handling of Data Integrity Observations DIO and Data Integrity Breach DIB. Without any confirmation of vials in system according the sequence dont start the run.
Data integrity breaches can result from poor practices or inadequate systemsprocedures. In its broadest use data integrity refers to the accuracy reliability and consistency of data stored over its entire life-cycle in a database data warehouse data mart or other construct. Data must be completed and accurate without any alteration.
Data Integrity Guidance in Pharmaceuticals. All pharmaceutical industry follows the good documentation practices for the consistency in documentation. The manufacturing and testing of pharmaceuticals is the biggest field wherein the accuracy data produced is strictly supervised by international guidelines such as.
Data should be complete and accurate without any alteration. Organisational culture behaviour encouraging to create right environment to implement effective Data integrity on site 3. Data Integrity In Pharmaceuticals Data integrity.
There is a stringent focus inside the global pharmaceutical industry and regulatory agencies to ensure data integrity. Systems to assure data quality and integritysystems should be designed in a way that encourages compliance with the principles of data integrity for examples include -. Data Integrity Handling of DI Observations DIO pharmabeginers.
This SOP is helpful to understand how the GDP plays an important role to minimize data integrity by following the ALCOA principle. Data integrity is a fundamental component of information security. Data Integrity in the Pharmaceutical Industry.
Effective Data Quality Stakeholders have the ultimate responsibility to ensure an effective pharmaceutical quality system is in place to achieve the quality objectives and that roles responsibilities and authorities are defined communicated and implemented throughout the. In the pharmaceutical industry ensuring data integrity involves generating and documenting data accurately protecting data from accidental or intentional modifications falsification deletion or destroying data. Regulatory agencies and the pharmaceutical industry need accurate and reliable data to ensure the safety efficacy and quality of products.
Access to clocks for recording timed events. All the data either in manual form or electronic must maintain its integrity. Data integrity DI ensures that the data generated during business operations and drug manufacturing is accurate complete and reliableISPE GAMP Guide.
The purpose of good documentation practices procedure to maintain data integrity. Uphold quality control data integrity by reducing human error. Data recorded as per GMP requirement and ensuring data is complete consistent and accurate throughout the lifecycle ie paper or electronic 2.
Its good learn about data integrity procedures. Audit cGMP Doc GLP QA Sop QC Sop Quality Control SOPs. Action must be.
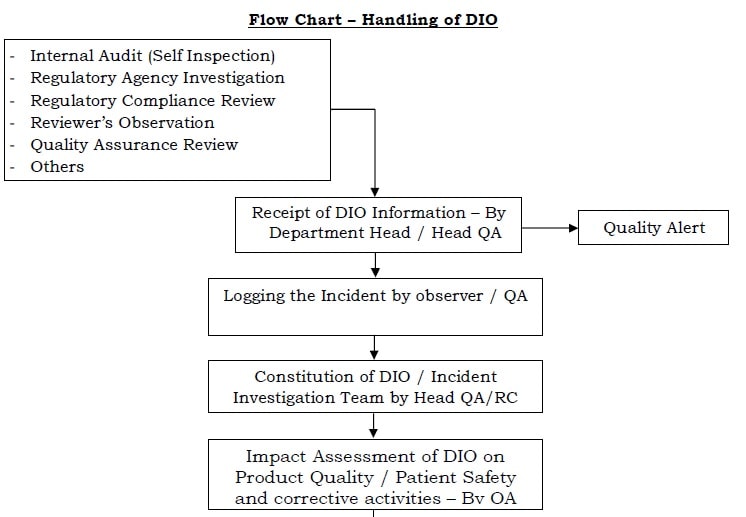
Data Integrity Handling Of Di Observations Dio Pharma Beginners
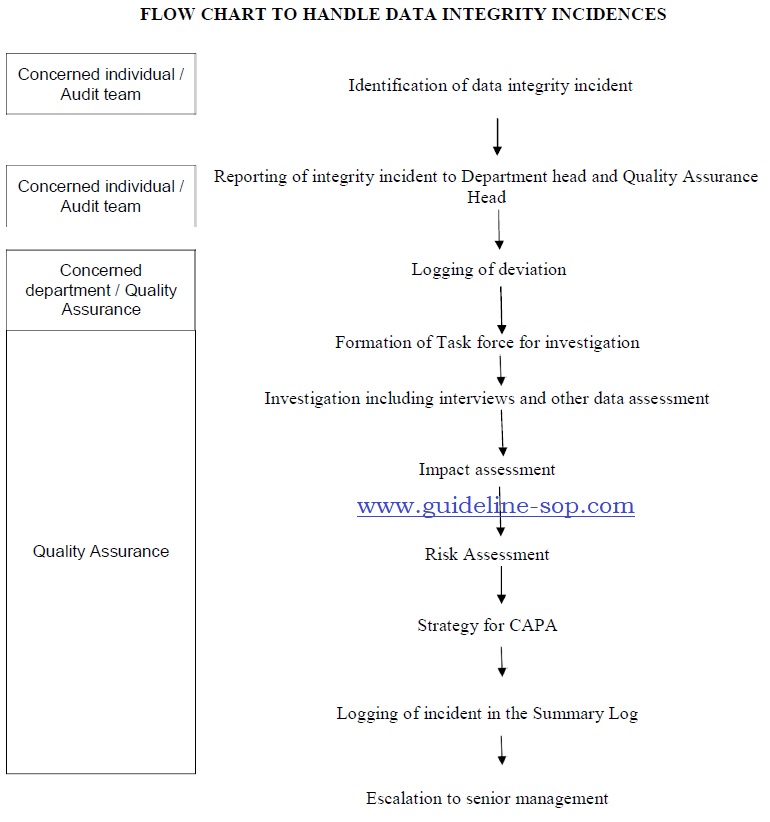
Data Integrity Incident Handling Procedure Guidelines Sops

Sop On Data Integrity In Pharmaceutical Industry
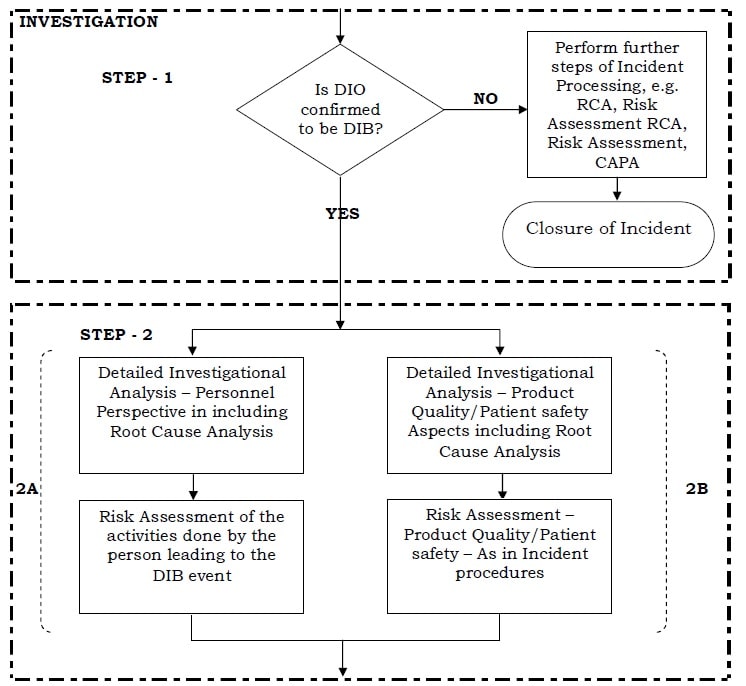
Data Integrity Handling Of Di Observations Dio Pharma Beginners
No comments for "Data Integrity Sop in Pharmaceutical Industry"
Post a Comment